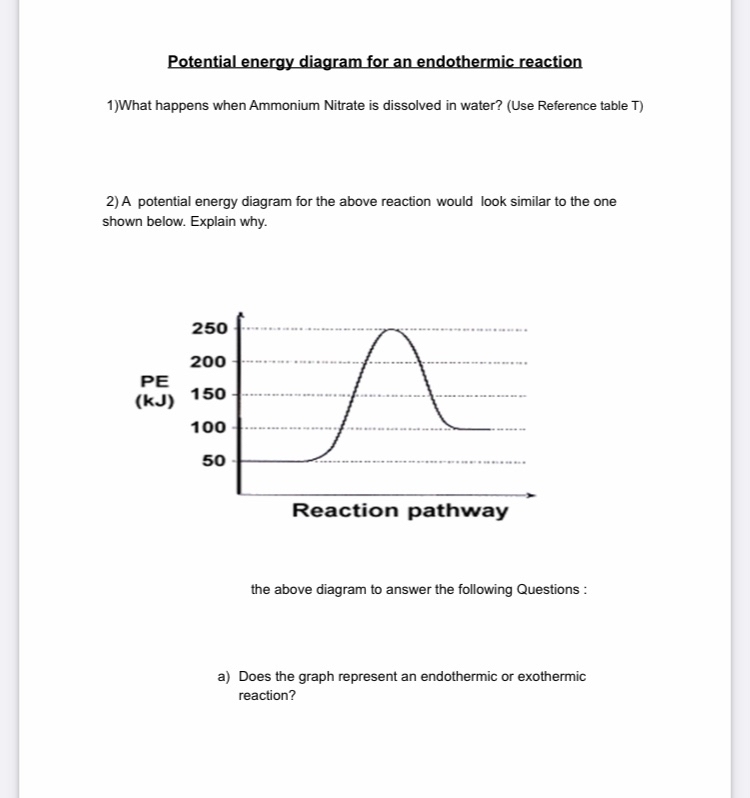
39 exothermic potential energy diagram
PDF Potential Energy Diagrams THE DIAGRAMS GRAPHICALLY COMPARE REACTANT ENERGY TO PRODUCT ENERGY AS REACTION OCCURS IF REACTANTS HAVE MORE ENERGY THEN PRODUCTS, THE REACTION IS EXOTHERMIC (loss of energy) IF REACTANTS HAVE LESS ENERGY THEN PRODUCTS, THE REACTION IS ENDOTHERMIC (gain of energy) ENDOTHERMIC OR EXOTHERMIC? Note the REACTANTS and PRODUCTS are labeled. Energy Diagrams of Reactions | Fiveable Energy Diagrams. Exothermic Reactions. Endothermic Reactions. Example. 6.3 Kinetic Energy, Heat Transfer, and Thermal Equilibrium. 6.4 Heat Capacity and Coffee-Cup Calorimetry. 6.5 Phase Changes and Energy. 6.6 Introduction to Enthalpy of Reaction. 6.7 Bond Enthalpy and Bond Dissociation Energy.
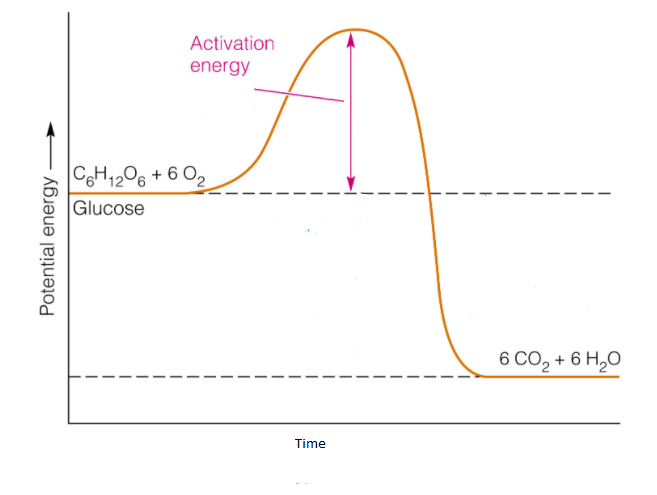
How to tell if a reaction is exothermic or endothermic ... Image of a graph showing potential energy in relation to the process of a chemical reaction. In the case of an exothermic reaction, the reactants are at a higher energy level as compared to the products, as shown below in the energy diagram. In other words, the products are more stable than the reactants.

Exothermic potential energy diagram
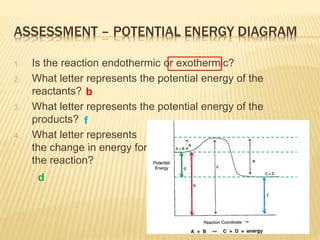
en.wikipedia.org › wiki › Energy_densityEnergy density - Wikipedia This energy, E = mc 2, where m = ρV, ρ is the mass per unit volume, V is the volume of the mass itself and c is the speed of light. This energy, however, can be released only by the processes of nuclear fission (0.1%), nuclear fusion (1%), or the annihilation of some or all of the matter in the volume V by matter-antimatter collisions (100%). Chemistry 30 Assessment Exemplars, 2020-2021 - Alberta A. endothermic, and the products have more potential energy than the reactants B. endothermic, and the reactants have more potential energy than the products C. exothermic, and the products have more potential energy than the reactants D. exothermic, and the reactants have more potential energy than the products Answer: D Outcome: A1.10k, A2.2k ... PDF Potential Energy Diagram Worksheet ANSWERS The reverse reaction is ____exothermic_____ (endothermic or exothermic). Reaction Rates and Potential Energy Diagrams 1. Chemical reactions occur when reactants collide. For what reasons may a collision fail to produce a chemical reaction? Not enough energy; improper angle. 2. If every collision between reactants leads to a reaction, what ...
Exothermic potential energy diagram. › reviews › ElectricElectric Circuits Review - Answers - Physics Classroom In the same manner, electric potential energy at a given location is charge-dependent. The more charge present at a location, the more repulsive (or attractive) effects and the greater the potential energy. But electric potential makes the amount of charge unimportant since it is the electric potential energy per charge. Now electric potential ... PDF NAME Kinetics Potential Energy Diagrams - ISD 622 Potential energy diagrams are used to represent potential energy changes that take place as time progresses during chemical reactions. The potential energy diagrams for the exothermic reaction between substances X and Y is shown below. The reaction progresses from stage 1 (reactants), through stage 2 (activated complex to), to stage 3 (products ... Energy - Wikipedia Energy is also transferred from potential energy to kinetic energy and then back to potential energy constantly. This is referred to as conservation of energy. In this isolated system , energy cannot be created or destroyed; therefore, the initial energy and the final energy will be … PDF Topic 5.1 Exothermic and Endothermic Reactions Heat and ... Decrease in kinetic energy - energy released - exothermic Energy level diagrams Chemical Potential Energy The chemical potential energy stored in the bonds gives us a measure of a substances energy level. The higher the energy, the more chemical energy is stored in its bonds. The reactants and products
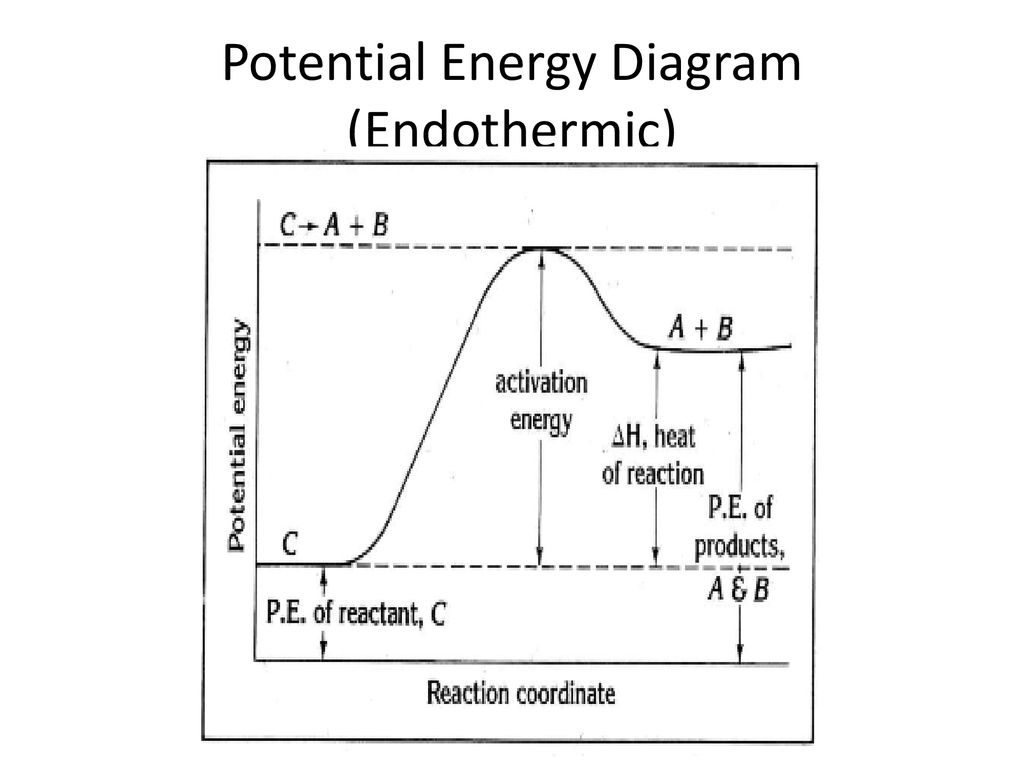
The Energy in Chemical Reactions: Thermodynamics and ... Objects can also possess potential energy. An object has potential energy because of its position in relation to other objects. When an object rises against the force of gravity, it gains potential energy; when it falls, it loses potential energy. The object stores this energy as it rises and releases it as it returns to Earth. Potential Energy Diagram Endothermic and Exothermic ... This potential energy diagram is used to identify the reaction as exothermic because we can see the value of the products being lower than the value of the reactants meaning the q value is negative. Generic Reactions Reactants Products Transition St ate Synthesis A B AB A B 15 kJ AB 20 kJ 30 kJ Single Replacement C AB CB A C AB 65 kJ CB A. Endothermic and Exothermic Activity.pdf - Endothermic and ... Endothermic and Exothermic Activity For this assignment, you will create your own potential energy diagrams for each of the three chemical reactions. Then you will analyze the data and your diagrams for each reaction. Generic Reactions Reactants Products Transition State Synthesis A + B → AB A + B −15 kJ AB 20 kJ 30 kJ Single Replacement C + AB → CB + A C + AB 65 kJ CB + A 30 kJ 85 kJ ... examples.yourdictionary.com › examples-of-chemicalExamples of Chemical Energy in Everyday LIfe 9 Examples of Chemical Energy. Chemical energy is either released (exothermic reaction) or absorbed (endothermic reaction) during a chemical reaction. In an exothermic reaction, heat is released, creating warmth. In an endothermic reaction, the heat is absorbed, creating cooling. Air bags are activated by a chemical reaction inside the bag. A ...
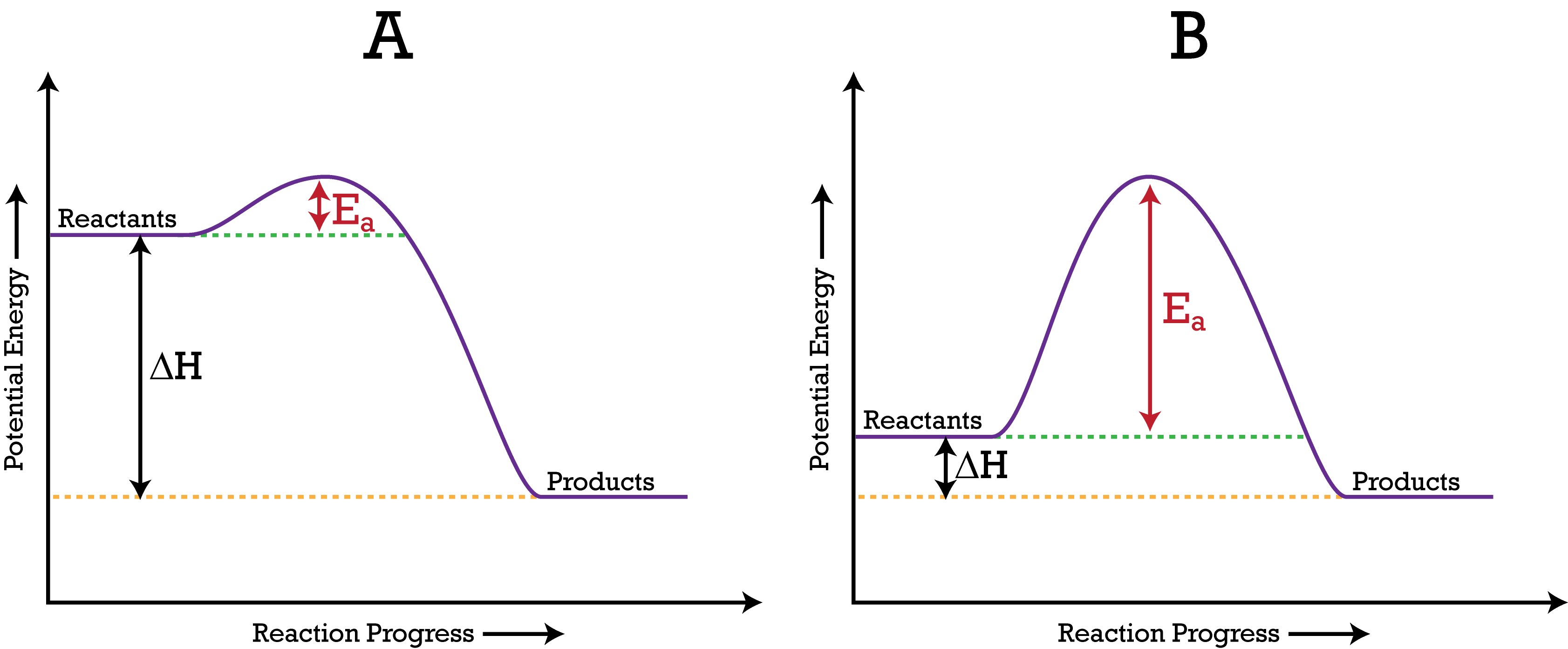
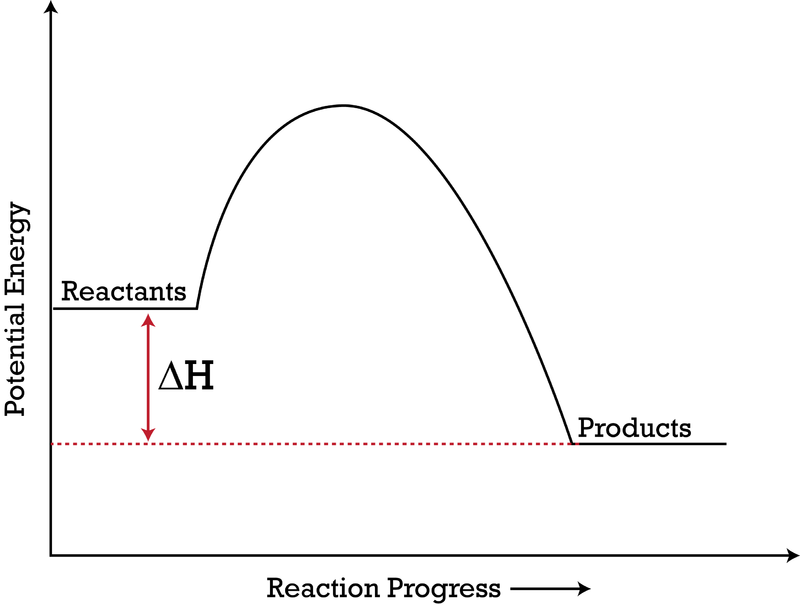
Answered: Consider the potential energy diagram… | bartleby Consider the potential energy diagram shown below. This graph shows the chemical potential energy in a reaction system over time. The y-axis is potential energy in kilojoules. The x-axis is the reaction progress, or time. Does this graph represent an endothermic or an exothermic reaction? Explain your answer. 18.4: Potential Energy Diagrams - Chemistry LibreTexts A potential energy diagram shows the change in potential energy of a system as reactants are converted into products. The figure below shows basic potential energy diagrams for an endothermic (A) and an exothermic (B) reaction. Recall that the enthalpy change ( Δ H) is positive for an endothermic reaction and negative for an exothermic reaction. Classify each chemical reaction as exothermic or ... Classify each chemical reaction as exothermic or endothermic. Potential energy diagram for the reaction Potential energy diagram for the reaction 24,0 + 572 kJ → 2H2 + O2 H₂.0₂ Potential Energy H,O Time o exothermic O endothermic DONE Energy Balances — Introduction to Chemical and ... Procedure for using the energy balance. Similar to those used in material balances, here are the recommended steps in solving problems in which energy balances are relevant: Draw a diagram if one is not already available. Write all known quantities (flow rates, densities, etc.) in the appropriate locations on the diagram.
Endothermic and Exothermic Reactions Diagram | Quizlet Always to the left of the arrow in a chemical equation. In exothermic reactions, there is more energy in the reactants than in the products. In endothermic reactions, there is less energy in the reactants than in the products.
Potential Energy Diagrams | Chemistry for Non-Majors A potential energy diagram shows the change in potential energy of a system as reactants are converted into products. The figure below shows basic potential energy diagrams for an endothermic (A) and an exothermic (B) reaction. Recall that the enthalpy change is positive for an endothermic reaction and negative for an exothermic reaction.
Fusion power - Wikipedia Fusion power is a proposed form of power generation that would generate electricity by using heat from nuclear fusion reactions.In a fusion process, two lighter atomic nuclei combine to form a heavier nucleus, while releasing energy. Devices designed to harness this energy are known as fusion reactors. Fusion processes require fuel and a confined environment with sufficient …
Potential Energy Diagrams - Kentchemistry.com Exothermic Reactions the reactants have more potential energy than the products have. The extra energy is released to the surroundings. Reactants --> Products + Energy Activated complex In this diagram, the activation energy is signified by the hump in the reaction pathway and is labeled.
Difference Between Endothermic and Exothermic Reactions ... The main difference between exothermic and endothermic reactions is that an endothermic reaction absorbs energy in the form of heat from its surroundings, whereas an exothermic reaction releases energy from its surroundings. Take up a quiz on Difference Between Endothermic and Exothermic Reactions. Q 5. Put your understanding of this concept to ...
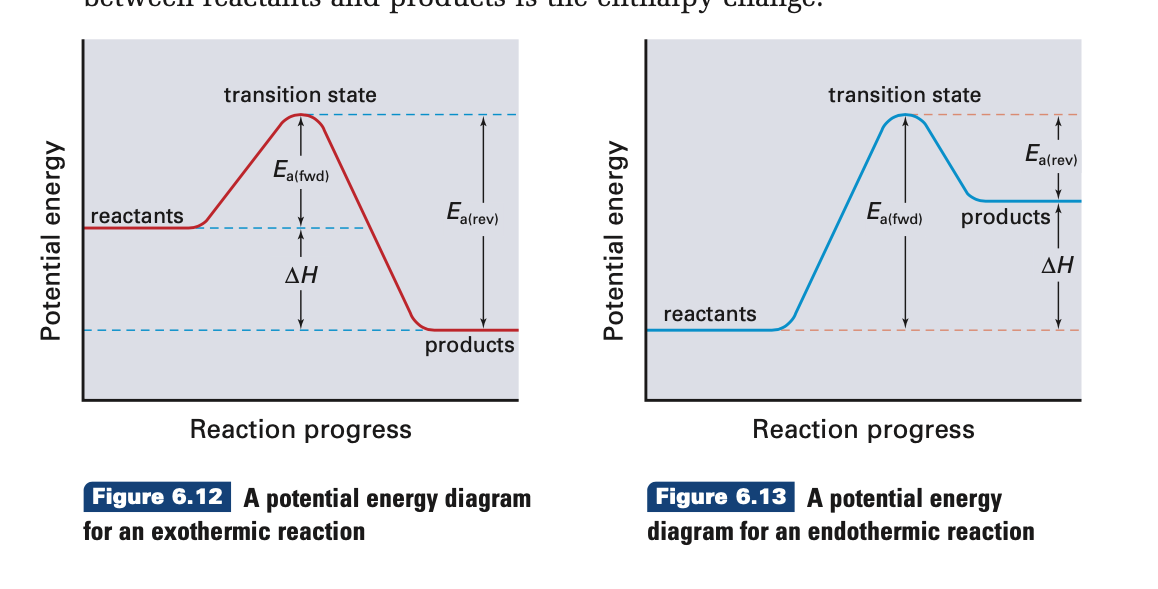
Representing endothermic and exothermic processes using ... The peaks in energy diagrams for both endothermic and exothermic reaction energy diagrams are known as the transition state or the activation complex. In a reaction, any reaction, the same general trend occurs. First the bonds of the reactants are broken which requires an input of energy to be put into the reaction.
Potential energy diagram with/without catalyst in a ... Potential energy diagram with/without catalyst in a hypothetical exothermic chemical reaction coordinate of Boltzmann distribution. The presence of the catalyst opens a different reaction pathway ...
Difference between Exothermic and Endothermic Reactions ... A chemical reaction that involves the release of energy in the form of heat or light is known as an exothermic reaction. When carbon burns in oxygen to make carbon dioxide, for example, a large amount of heat is produced. C + O2 → CO2 (Carbon) (Oxygen) (Carbon dioxide)
Solved Select the potential energy diagram that represents ... Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. Exothermic reactions are characterized by negative change in …. View the full answer. Transcribed image text: Select the potential energy diagram that represents an exothermic (exergonic) reaction.
3.15: Exothermic and Endothermic Processes - Chemistry ... Figure 3.15. 3: A potential energy diagram shows the total potential energy of a reacting system as the reaction proceeds. (A) In an endothermic reaction, the energy of the products is greater than the energy of the reactants and ΔH is positive. (B) In an exothermic reaction, the energy of the products is lower than the energy of the reactions ...
Welcome to CK-12 Foundation | CK-12 Foundation FlexBook Platform®, FlexBook®, FlexLet® and FlexCard™ are registered trademarks of CK-12 Foundation.
Potential Energy Diagrams - Chemistry - Catalyst ... This chemistry video tutorial focuses on potential energy diagrams for endothermic and exothermic reactions. It also shows the effect of a catalyst on the f...
Gibbs Free Energy - Purdue University Reactions are classified as either exothermic (H < 0) or endothermic (H > 0) on the basis of whether they give off or absorb heat. Reactions can also be classified as exergonic (G < 0) or endergonic (G > 0) on the basis of whether the free energy of the system decreases or increases during the reaction.. When a reaction is favored by both enthalpy (H o < 0) and entropy (S o > …
Endothermic vs Exothermic and Energy Diagrams.pdf - Name ... Key things to Note about ENDOTHERMIC Energy Diagram: Reactants have LESS potential energy compared to Products Energy was absorbed during the reaction (this is why the products have more energy) Δ H will be positive
[Solved] Please refer to the attachment to answer this ... The potential energy diagrams demonstrate this. As the system absorbs energy from the environment, its total potential energy rises for the endothermic reaction. For the exothermic reaction, the total potential energy of the system falls as the system releases energy into the environment. Part 2. Change in enthalpy
Endothermic and Exothermic Reactions With Potential Energy ... This chemistry video tutorial provides a basic introduction into endothermic and exothermic reactions as well as the corresponding potential energy diagrams....
What is Enthalpy? - Definition, Endothermic & Exothermic ... The cumulative energy that is stored in the device is internal energy. It is the amount held by the mechanism of potential and kinetic energy. Enthalpy is specified as the amount of the system’s internal energy plus the combination of the system’s gas pressure and its length.
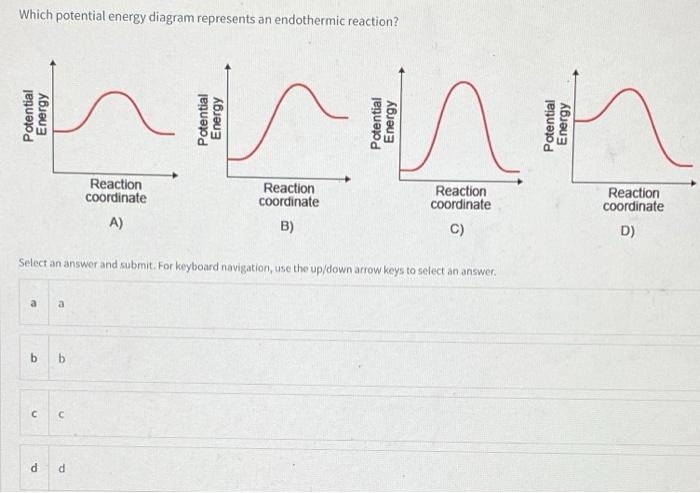
Solved Which potential energy diagram represents an ... Question: Which potential energy diagram represents an exothermic reaction? Potential Energy Potential Energy non Reaction coordinate A) Reaction coordinate B) Reaction coordinate C) Reaction coordinate D) Select an answer and submit. For keyboard navigation, use the up/down arrow keys to select an answer. a a b b с c d d Which potential ...
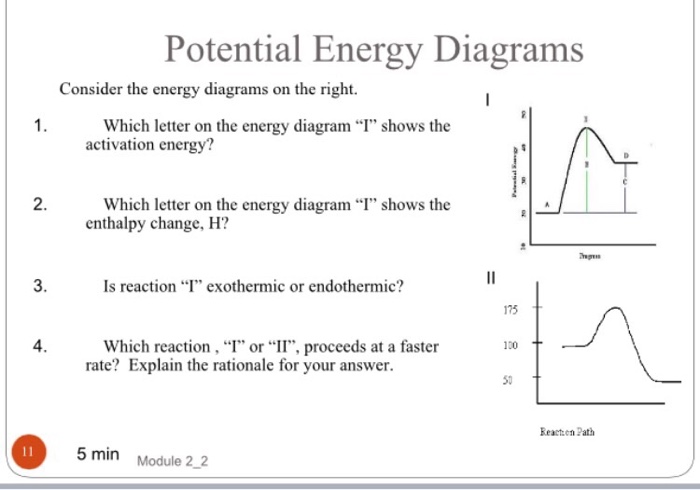
Welcome to CK-12 Foundation | CK-12 Foundation Look at the two exothermic reactions whose potential energy diagrams are represented in figures A and B below, and notice the activation energy marked in each. When a reaction has a low activation energy, like in figure A above, most of the reactant molecules have sufficient kinetic energy to react, and the reaction will most likely be rapid (a ...
How to draw the potential energy diagram for this reaction ... Identify the general shape of the energy diagram Energy should conserve for any chemical reaction. The reaction in question is exothermic (releases heat) hence its products shall have chemical potential energies lower than that of its reactants- some of the potential energies have been converted to thermal energy during the reaction process.
PDF Potential Energy Diagram Worksheet ANSWERS The reverse reaction is ____exothermic_____ (endothermic or exothermic). Reaction Rates and Potential Energy Diagrams 1. Chemical reactions occur when reactants collide. For what reasons may a collision fail to produce a chemical reaction? Not enough energy; improper angle. 2. If every collision between reactants leads to a reaction, what ...
Chemistry 30 Assessment Exemplars, 2020-2021 - Alberta A. endothermic, and the products have more potential energy than the reactants B. endothermic, and the reactants have more potential energy than the products C. exothermic, and the products have more potential energy than the reactants D. exothermic, and the reactants have more potential energy than the products Answer: D Outcome: A1.10k, A2.2k ...
en.wikipedia.org › wiki › Energy_densityEnergy density - Wikipedia This energy, E = mc 2, where m = ρV, ρ is the mass per unit volume, V is the volume of the mass itself and c is the speed of light. This energy, however, can be released only by the processes of nuclear fission (0.1%), nuclear fusion (1%), or the annihilation of some or all of the matter in the volume V by matter-antimatter collisions (100%).


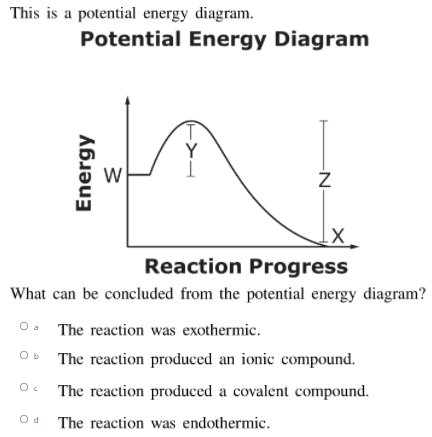







![SOLVED:[KIU] Based on the potential energy diagram shown ...](https://cdn.numerade.com/ask_images/fc05665f445f430d9f3e74676368bd48.jpg)











Comments
Post a Comment