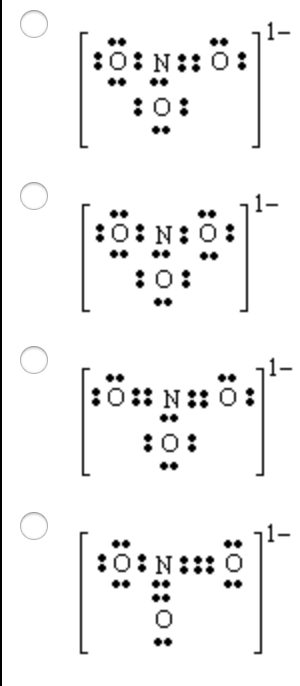
40 lewis dot diagram for no3
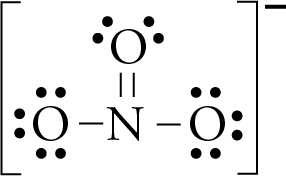
A step-by-step explanation of how to draw the NaNO3 Lewis Dot Structure.For NaNO3 we have an ionic compound and we need to take that into account when we dra... In lewis structure of NO 3- ion, there are three lone pairs (in the last shell) in two oxygen atom and that oxygen atoms. Also, those two oxygen atoms has a -1 charge. There is another oxygen atom. That oxygen atom is connected to the nitrogen atom by a double bond has two lone pairs in its last shell. Also, there is no charge in that oxygen atom.
Answer (1 of 2): You draw the Lewis dot structure for NOCl in exactly the same way as you'd do it for any simple, covalently bonded compound. First you need to know the valency of each element. Then you need to draw a stick model with bonds between the atoms, so that each atom's valency is satis...
Lewis dot diagram for no3
Answer (1 of 2): For the NO3- Lewis structure, calculate the total number of valence electrons for the NO3- molecule. After determining how many valence electrons there are in NO2, place them around the central atom to complete the octets. In the Lewis structure of NO3- there are a total of 24 v... A step-by-step explanation of how to draw the NO3- Lewis Dot Structure (the Nitrate ion).For the NO3 - structure use the periodic table to find the total num... A step-by-step explanation of how to draw the Zn(NO3)2 Lewis Dot Structure.For Zn(NO3)2 we have an ionic compound and we need to take that into account when ...
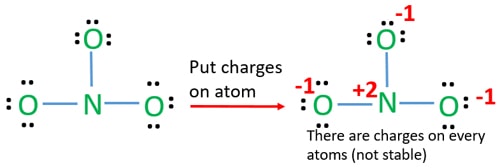
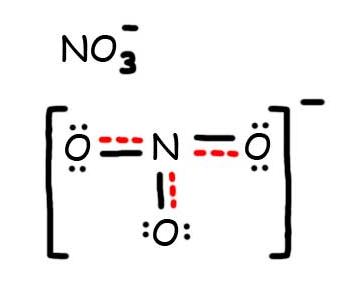
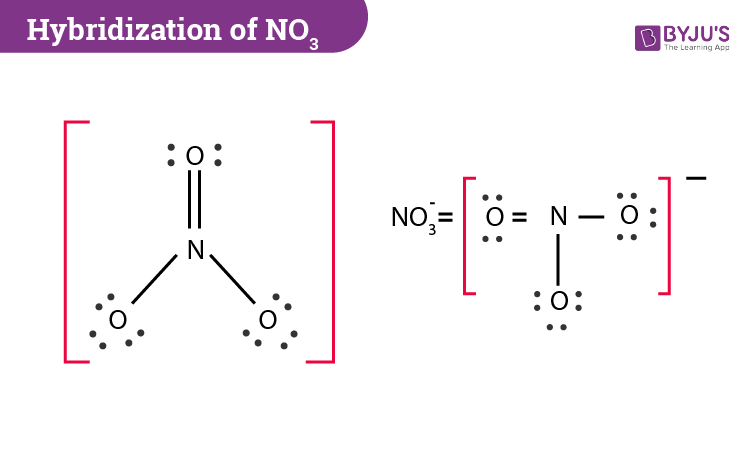
Lewis dot diagram for no3. Drawing the Lewis Structure for NO 3- (Nitrate Ion) Viewing Notes: The Lewis structure for NO 3- (Nitrate Ion) comes up quite often in chemistry. Be sure to put brackets, along with a negative sign, around the NO 3- Lewis structure when you are done to show that it is an ion with a negative charge. NO 3- has a total of 24 valence electrons. Well, you gots a potassium cation, and nitrate anion as the gegenion.. Nitrate anion is an interesting customer in terms of resonance, in that THREE of the FOUR atoms bear a formal electric charge in the resonance structure. And of course the charges SUM to the formal charge of nitrate anion, i.e. negative ONE... And so for NO_3^(-)...we add up the valence electrons, i.e. 5_"nitrogen electrons ... The Lewis Structure (electron dot diagram) of each ion is used to construct the Lewis Structure (electron dot diagram) for the ionic compound. The Lewis structure of a positive ion (cation) is positioned adjacent to the Lewis structure of a negative ion (anion). If the charge on the positive ion is greater than the charge on the negative ion ... The Lewis representation of NO3 is aligned in a way where the nitrogen atom is in the center and orbited by three oxygen atoms. The nitrogen has a positive charge because it has 4 bonding electrons - 2 from the oxygen double bond and 1 from each of the N - O bonds.
Answer : The correct Lewis-dot structure of nitrate ion is shown below and the nitrogen has 4 covalent bonds. Lewis-dot structure : It shows the bonding between the atoms of a molecule and it also shows the unpaired electrons present in the molecule. The number of valance electrons are shown by 'dot'. The Lewis structure for nitrogen trioxide starts with an N atom in the center. To the left is a doubly bonded O atom with two pairs of dots. At the top and right is a singly bonded O atom with ... In the Lewis structure for NO2 the Nitrogen atom is the least electronegative atom and goes at the center of the structure. For the NO2 Lewis structure, calculate the total number of valence electrons for the NO2 molecule. After determining how many valence electrons there are in NO2, place them around the central atom to complete the octets. Lewis Electron-Dot Structure for NO3. Chemistry. Instructional. An Introduction to Chemistry. General Chemistry Modules. General Chemistry Problems.
Write Lewis dot diagrams for CO2, NO3 and CO3 as well as indicate the resonance forms if available. Lewis Structures Lewis structures are also known as electron dot structures. The structures show... Answer (1 of 2): Well, I think you mean \text{nitrate ion}, the which is NO_{3}^{-} …. And here we got 5+3×6+1=24•\text{valence electrons} to distribute across 4 centres … and thus we get a quaternized nitrogen atom with a formal positive charge, and TWO of the oxygen atoms have a formal negativ... I quickly take you through how to draw the Lewis Structure of NO3- (Nitrate Ion). I also go over the resonance, hybridization, shape and bond angle. What is the lewis structure for NO3 -1? The Lewis structure begins with an N atom in the center with two O atoms singly bonded. Each of these O atoms has three pair of dots on the unbonded sides....
Construction of NO3 Lewis Dot Structure. 1. In the ion NO3, there is 1 atom of nitrogen and 3 atoms of oxygen. It also has one negative charge. 2. Nitrogen and oxygen belong to periods 5A and 6A groups respectively in the periodic table. Hence, oxygen has 6 and nitrogen has 5 valence electrons in their outer shell. 3.
Lewis Structure of NO 3-(Nitrite ion). Lewis structure of NO 3-ion is drawn step by step in this tutorial. Total valence electrons of nitrogen and oxygen atoms and negative charge are considered to draw the NO 3-lewis structure. You will every fact of drawing lewis structures from this tutorial which will help you to draw more lewis structures in the future.
Solved Draw The Lewis Structure For The Following A H2cno2 With C And N As The Central Atoms B N2o C No3 D Co32 E Scn F No G Course Hero
2. Draw the Lewis dot structures for each of the following molecules: a. H 2 S c. SO 3 b. CH 2 Br 2 d. HCN 3. Draw the Lewis dot structure for each of the following polyatomic ions: a. NH 4 + c. PO 4 -3 b. NO 3 - d. CO 3 2- 4. For the following molecules or ions (where the central atom is underlined): i. Draw the Electron dot structure. ii.
The Lewis dot diagram for a Ge− G e − anion is shown below. A neutral Germanium atom has 4 valence electrons. Hence, a Ge− G e − anion which a charge of -1 has a total of 5 valence electrons.
Construction of NO3 Lewis Dot Structure. 1. In the ion NO3, there is 1 atom of nitrogen and 3 atoms of oxygen. It also has one negative charge. 2. Nitrogen and oxygen belong to periods 5A and 6A groups respectively in the periodic table. Hence, oxygen has 6 and nitrogen has 5 valence electrons in their outer shell. 3. Notice the number of ...
A step-by-step explanation of how to draw the NO3- Lewis Structure (Nitrate Ion). Get more chemistry help at www.Breslyn.org.For the NO3- Lewis structure,...

Write The Various Steps Involved In The Lewis Structure For Nitrate No 3 Ion Sarthaks Econnect Largest Online Education Community
Drawing the Lewis Structure for NO 3-(Nitrate Ion). Nitrates (salts with NO 3-) are frequently used in agriculture as a fertilizer.This is in part to their high solubility in water. There are 24 valence electrons available for the Lewis structure for NO 3-.. Video: Drawing the Lewis Structure for NO 3-
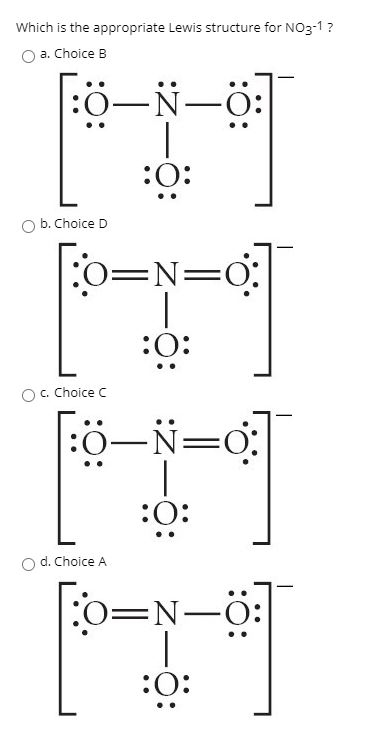
Lewis Dot of the Nitrate Ion. NO 3-. Back. 70 More Lewis Dot Structures. The nitrate ion cannot be satisfactorily represented by just one Lewis Dots structure. All the bonds are the same length and must be thought of as a hybrid of multiple resonance structures. YouTube.
Lewis dot structures| NO3- ion | nitrate ion | method to draw dot structure| on line textbook,lewis structure of no3- with formal charges, lewis structure of no3- molecule, Lewis structure, resonance structures of no3-, resonance structures of no3-1, resonance lewis structure of no3-, lewis dot structures of no3-, no3-1 lewis dot structure, no3 electron dot structure, lewis dot structure of ...
Problem: There are 3 Lewis structures for [ NO3]- draw all three and compare polarity of the 3. FREE Expert Solution. 83% (96 ratings) Problem Details. There are 3 Lewis structures for [ NO 3]-draw all three and compare polarity of the 3. Learn this topic by watching Molecular Polarity Concept Videos.
A step-by-step explanation of how to draw the Zn(NO3)2 Lewis Dot Structure.For Zn(NO3)2 we have an ionic compound and we need to take that into account when ...
A step-by-step explanation of how to draw the NO3- Lewis Dot Structure (the Nitrate ion).For the NO3 - structure use the periodic table to find the total num...
Answer (1 of 2): For the NO3- Lewis structure, calculate the total number of valence electrons for the NO3- molecule. After determining how many valence electrons there are in NO2, place them around the central atom to complete the octets. In the Lewis structure of NO3- there are a total of 24 v...

Draw Lewis Structures For No2 No2 And No3 Based On The Interpretation Of The Lewis Structures Put These In Order Of Increasing Bond Order Study Com
A Draw Lewis Structures For Co2 So2 And No3 B Give The Electron Pair Geometry And The Molecular Geometry Of The Three Species From Part A According To Vsepr C Are Co2

Calculating No3 Formal Charges Calculating Formal Charges For No3 Teaching Chemistry Chemistry Classroom Science Chemistry




















Comments
Post a Comment