39 penny battery diagram
Batteries. In the beginners tutorials on this website, we use a nine volt (9V) battery with the breadboard circuits. A single 9V battery or a battery holder containing six 1.5V cells can be used. Either of these batteries needs a battery clip (connector) which has two wires for connecting the battery to the circuit. A lithium-ion battery is an energy storage device providing electrical energy by using chemical reactions. A few types of lithium-ion battery cells have been used widely as shown in Figure 1. With the cylindrical cell format, the batteries can be applied to many applications, for example, power tools, laptops,
Silver Oxide Battery Alkaline Battery Lithium Manganese High 11.6 5.4 1.55 165 SR44W 357 D357 357 357(7) V357(541) SB-B9 - J SR1154PW Drain 4.2 1.55 125 SR43W 386 D386 386 386(6) V386(548) SB-B8 280-41 H SR1142PW Watch 3.05 1.55 79 SR1130W 389 D389 389 389(17) V389(554) SB-BU 280-15 M SR1130PW

Penny battery diagram
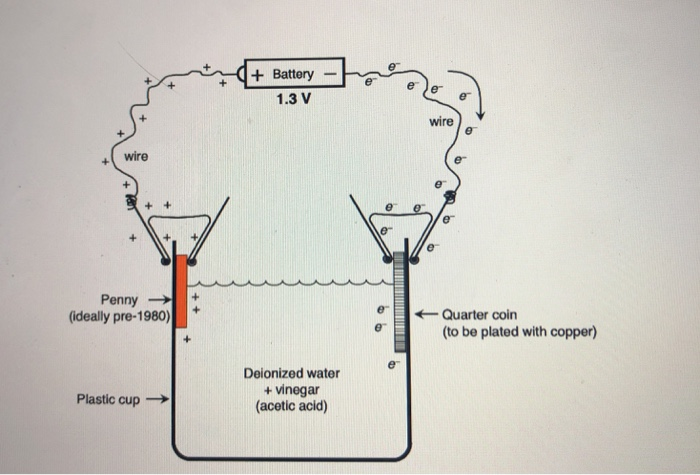
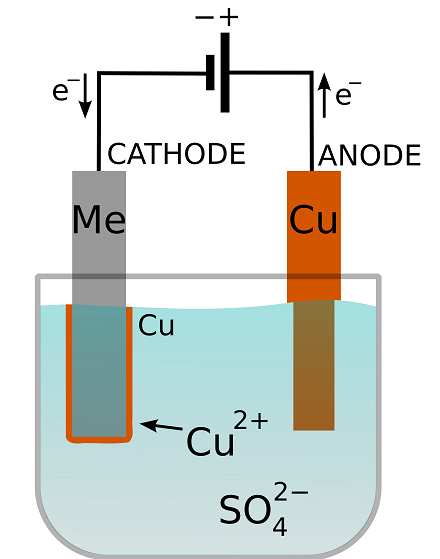
In this experiment (Figure 1), a U.S. penny acts as the copper source/anode and a U.S. dime serves as the cathode (a dime, versus another penny, makes the movement of copper more obvious). A dilute aqueous solution of copper sulfate and sulfuric acid is used as the electrolyte. An electric current is provided by a 9 volt (V) battery. 1.5 Remove coin cell battery tab 1.5a Remove tab to activate coin cell battery. NOTE: Coin cell battery saves time and date during a power loss. 1.6 Mount thermostat on wallplate. Align thermostat at bottom and snap into place as shown. REMOVE DURING INSTALLA REMOVE DURIN TION G INST ALLA TION Thermostat Coin cell battery tab Wallplate Building and Testing a Penny Battery: This version of a battery hearkens back to the original voltaic pile invented by Alessandro Volta in 1799. Here I will be showing two versions using different anode materials. In the diagrams I've only shown batteries composed of 5 cells, but the pi…
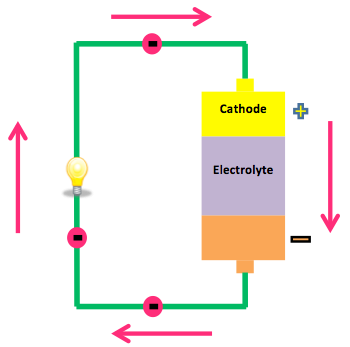
Penny battery diagram. and the voltage response of the battery in presence of variable input current. ... Figure 2 Schematic diagram of a Li-ion cell [7] ... • Cycle coin cells and measure relevant parameters including capacity, resistance, state of charge and open-circuit voltage, to understand the charge and discharge ... Our comprehensive equipment manuals are your one-stop resource for vital information on every Henny Penny product. To find the manual you're looking for, simply check the appropriate category/categories below to search, then continue through the resource finder to find a list of available manuals. Simple diagram to allow backup domain with minimum external component: diagram allowing longer battery lifetime Diagram for applications implementing USB Diagram for IoT or mobile application using only coin cells Low power diagram for IoT Different possibilities are proposed, showing the trade-off between lifetime and cost. www.st.com A potato battery is a type of battery that is known as an electrochemical cell. The chemicals zinc and copper (in the screw and penny/wire) react with each other, which produces chemical energy. This chemical energy is converted to electric energy by a spontaneous electron transfer. The potato acts as a buffer and an electrolyte for the two metals.
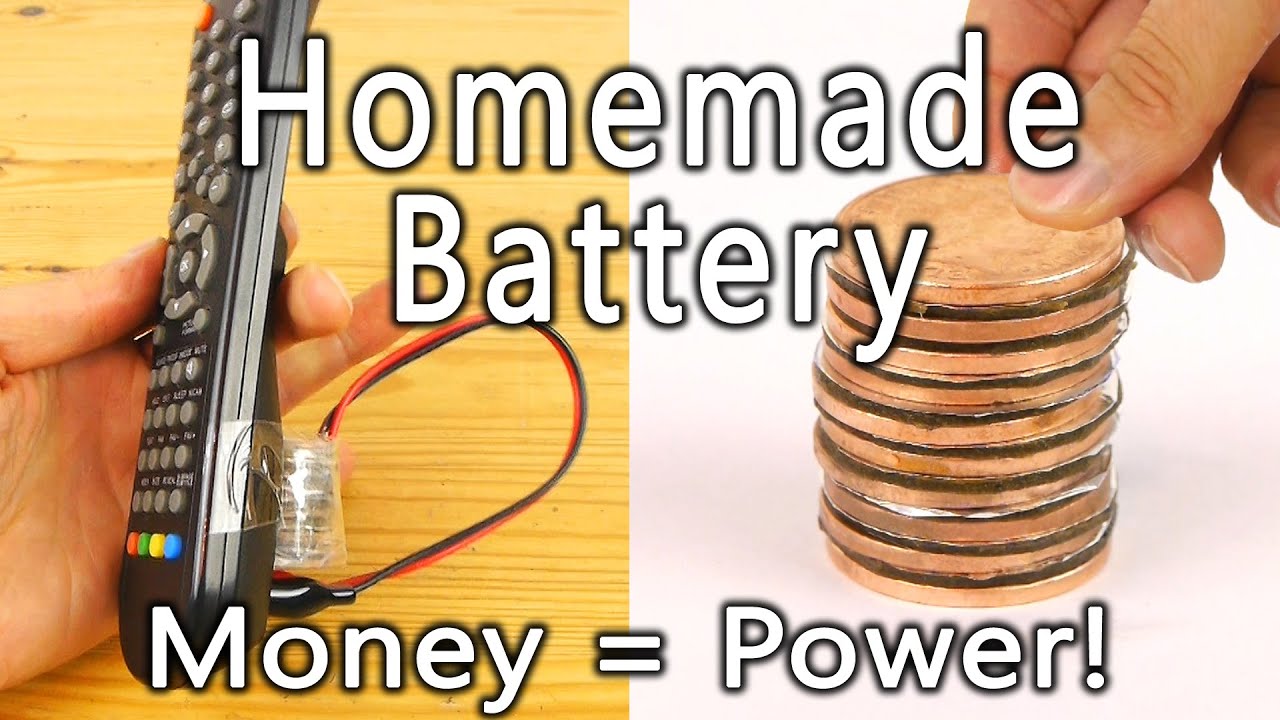
For an added challenge, try making a battery powerful enough to light a blue LED. Before 1982, pennies were made of 95% copper, but the rising costs of copper led the United States Mint to change the composition of the penny. The metal content in a pre-1982 penny is actually worth more than its one-cent face value. the large number of distributed nodes possible in such a system, battery operated nodes designed with cost-effective, nanopower, electronic components are desirable. Typically, the wireless nodes need to run on a single CR2032 coin battery for eight to ten years. The block diagram of a wireless, battery-powered What is a battery? A battery is a self-contained, chemical power pack that can produce a limited amount of electrical energy wherever it's needed. Unlike normal electricity, which flows to your home through wires that start off in a power plant, a battery slowly converts chemicals packed inside it into electrical energy, typically released over a period of days, weeks, months, or even years. The penny battery is a voltaic pile which uses various coinage as the metal disks (pennies) of a traditional voltaic pile. The coins are stacked with pieces of electrolyte soaked paper in between (see diagram at right). The penny battery experiment is common during electrochemistry units in an educational setting.. Each cell in a penny battery can produce up to 0.8 volt, and many can be ...
This battery comes in various forms and is of great use in a variety of applications, for example, a coin type for digital clocks, a pack type for cameras, and a pin type for fishing floats. Alkaline button battery. Nominal voltage 1.5 V. ... Let's take a look at this simple diagram. The molecular formula for water is H2O. This means that it ... The battery will run until the liquid dries up or the chemical reaction dies. DIY enthusiast Grant Thompson recently made a 10-penny battery that powered a small light for nearly two weeks. See ... The two coin cells shown are each 1.5 volts. A typical LED needs 3 volts to emit light effectively. The circuit diagram is shown below. The battery, switch and LED symbols have been included. Although no switch is used in reality, the switch mimics the two fingers pressing the legs of the LED together against the batteries. ... The coin type lithium manganese dioxide battery uses manganese dioxide (MnO2) as its positive active material, lithium (Li) as its negative active material, and an organic electrolyte solution. ˜ Optimum for Memory and RTC Backup (Fig. 1) Displays long-term stable operating voltage at low load discharge.
Figure 1 shows a schematic diagram of a circuit which will fast-charge a 12V Ni-Cd or Ni-MH battery at 2.6A and trickle charge it when the converter is shut off. Note that the circuit must have a shutdown pin so that the end-of-charge detection cir-cuit(s) can terminate the fast charge cycle when the battery is full (the LM2576 has a
Panasonic coin type lithium batteries are high energy, high reliability batteries for a variety of applications. The full 3V in these high energy batteries is about twice that of conventional dry batteries.
To generate electrical energy, this battery relies on oxidation of aluminum at the anode, which releases electrons, and a reduction of oxygen at the cathode, which uses electrons. ... A diagram of the battery and equations for the half and overall reactions are given below: ... Penny Battery. Light an LED with five cents.
A penny battery uses the copper and zinc in a penny along with some acid to make a battery. ... Here are the dimensions of a U.S. penny. I have also included a diagram showing how they could be ...
Anatomy of a Battery. Take a look at any battery, and you'll notice that it has two terminals. One terminal is marked (+), or positive, while the other is marked (-), or negative. In normal flashlight batteries, like AA, C or D cell, the terminals are located on the ends. On a 9-volt or car battery, however, the terminals are situated next to ...
4. Tuck the penny half-way into the slit on each lemon. The penny is the cathode. 5. Press one nail into each of 7 lemons. The nail is the anode. Connect the battery to the bulb 6. Connect one end of an alligator clip to the zinc nail (anode). Connect the other end of the alligator clip to the penny copper penny (cathode) of a different lemon. 7.
Batteries joined together in Series: have the effect of doubling the voltage, the Ampere Hour stays constant as the diagram above using identical batteries (of the same voltage and Ampere-hours) shows. Configuration: 2 x 60Ah connected in series = 24V 60Ah output. Ampere-Hour (Ah): The time that a battery can deliver (in an hour) the stated current (in Amperes), or the electric charge ...

A Review Of Rechargeable Batteries For Portable Electronic Devices Liang 2019 Infomat Wiley Online Library
Replacing a WW Scale Battery. Your WW Scale by Conair ™ is powered by one of two different types of batteries: lithium coin battery (CR2032) or alkaline batteries (AAA).. CR-2032 - 3 volt lithium battery; AAA - 1.5 volt alkaline dry-cell battery; When it's time to replace your WW scale batteries, the screen on your scale will display the word Lo.To replace the batteries, flip the scale ...
Steps To Be Followed to Build a Potato Battery. First, strip one of the ends of one copper wire, and if you have the penny available, then fit the penny into the stripped end. Cover the entire penny with the stripped end of the copper wire and make a tight fit.
To estimate the battery diameter, compare the battery with a U.S. penny (19 mm) and nickel (21 mm). Consult the National Battery Ingestion Hotline at 800-498-8666 for assistance in battery identification and patient management. If the patient is ≤ 12 years, immediately obtain an x-ray to locate the battery.
Frequently, OEMs design the battery cavity of their device around the battery of a single manufacturer. Unfortunately, battery dimensions often vary from manufacturer to manufacturer. For instance, the height of one manufacturer's 9-volt battery is 1.888 inches, while another's 9-volt battery measures 1.909 inches. While these
Building and Testing a Penny Battery: This version of a battery hearkens back to the original voltaic pile invented by Alessandro Volta in 1799. Here I will be showing two versions using different anode materials. In the diagrams I've only shown batteries composed of 5 cells, but the pi…
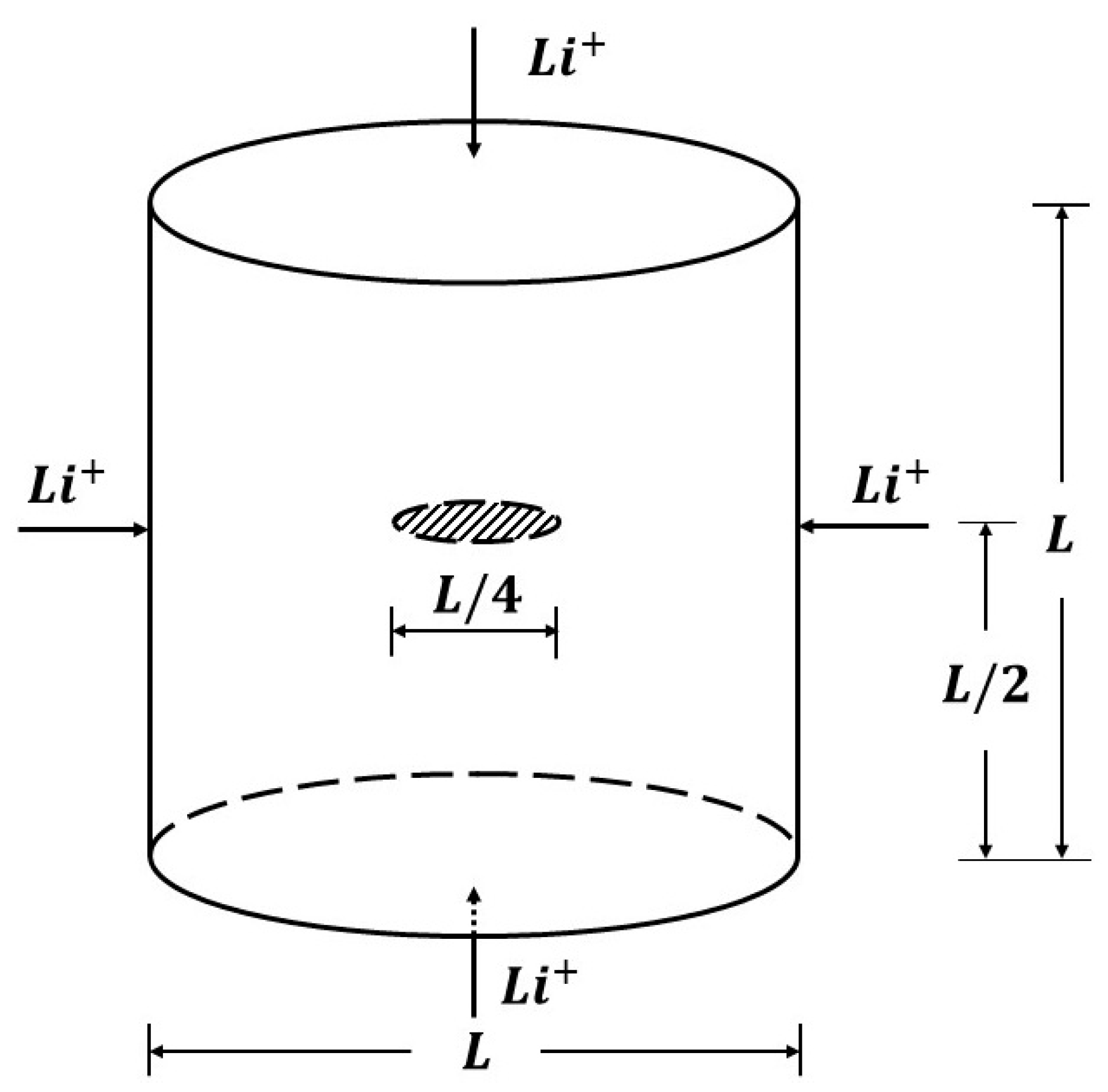
Energies Free Full Text Three Dimensional Peridynamic Model For Predicting Fracture Evolution During The Lithiation Process Html
1.5 Remove coin cell battery tab 1.5a Remove tab to activate coin cell battery. NOTE: Coin cell battery saves time and date during a power loss. 1.6 Mount thermostat on wallplate. Align thermostat at bottom and snap into place as shown. REMOVE DURING INSTALLA REMOVE DURIN TION G INST ALLA TION Thermostat Coin cell battery tab Wallplate
In this experiment (Figure 1), a U.S. penny acts as the copper source/anode and a U.S. dime serves as the cathode (a dime, versus another penny, makes the movement of copper more obvious). A dilute aqueous solution of copper sulfate and sulfuric acid is used as the electrolyte. An electric current is provided by a 9 volt (V) battery.

Rough Science Rough Science Adventure Island Power Plant School Science Experiments Science For Kids Science

















Comments
Post a Comment