39 hydrogen atom energy level diagram
Atomic structure concepts ... The basic hydrogen energy level structure is in agreement with the Bohr model. ... Electron energy level diagrams ... The ground state corresponds to the lowest energy level (n = 1). This is the most stable energy level. For the hydrogen atom (1 proton, 1 electron), its value ...
Energy level diagrams and the hydrogen atom. It's often helpful to draw a diagram showing the energy levels for the particular element you're interested in.
Hydrogen atom energy level diagram
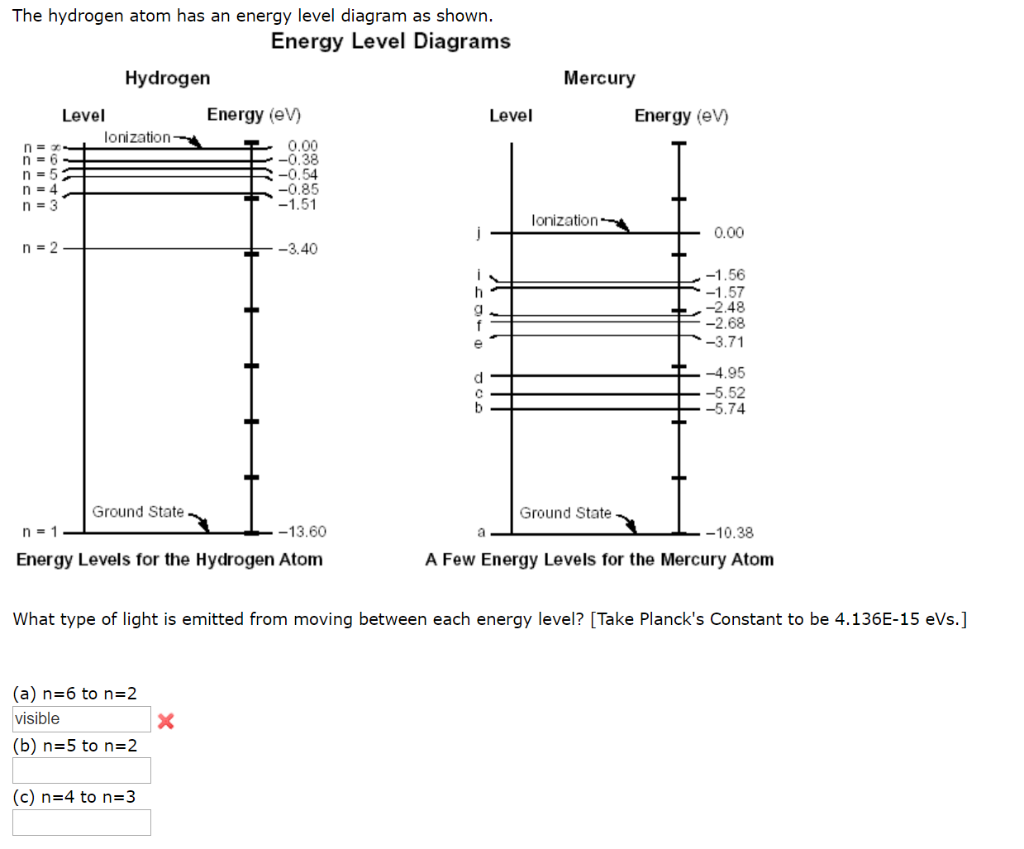
21 Nov 2010 — Energy Level Diagram For Hydrogen · n represents the principle quantum number and only takes integral values from 1 to infinity. · The ground ... A Hydrogen atom has only one single electron revolving around the nucleus at the lowest energy level. When we heat a hydrogen atom, the electron gains energy ...1 answer · Top answer: Hint: Energy level diagram is the direct consequence of the principal quantum number - ‘n’. Energy diagram is necessary to determine the energy difference ... Click here to get an answer to your question ✍️ Draw a neat labelled energy level diagram of the Hydrogen atom.19 Nov 20191 answer · Top answer: Given figure shows energy level diagram for Hydrogen atom.
Hydrogen atom energy level diagram. How Bohr's model of hydrogen explains atomic emission spectra. ... be illustrated using an energy level diagram, such as the example above showing electrons ... 29 Mar 2021 — Bohr model of the atom: electron is shown transitioning from the n=3 energy level to the n=2 energy level. The photon of light that is emitted ... Click here to get an answer to your question ✍️ Draw a neat labelled energy level diagram of the Hydrogen atom.19 Nov 20191 answer · Top answer: Given figure shows energy level diagram for Hydrogen atom. A Hydrogen atom has only one single electron revolving around the nucleus at the lowest energy level. When we heat a hydrogen atom, the electron gains energy ...1 answer · Top answer: Hint: Energy level diagram is the direct consequence of the principal quantum number - ‘n’. Energy diagram is necessary to determine the energy difference ...
21 Nov 2010 — Energy Level Diagram For Hydrogen · n represents the principle quantum number and only takes integral values from 1 to infinity. · The ground ...
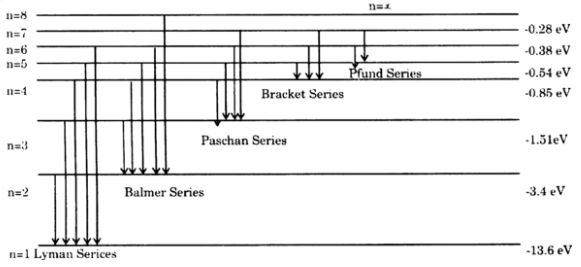
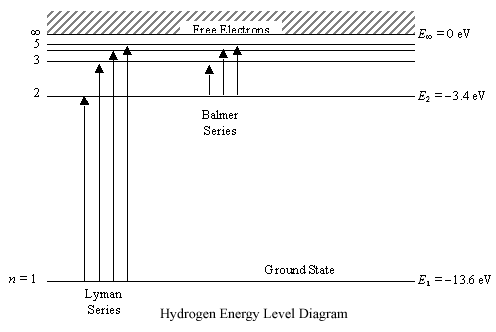
On An Energy Level Diagram Of Hydrogen Show By A Downward Or An Upward Arrow A Transition Which Results In I Emission Line Of Balmer Series Ii Emission Line Of Lyman Series

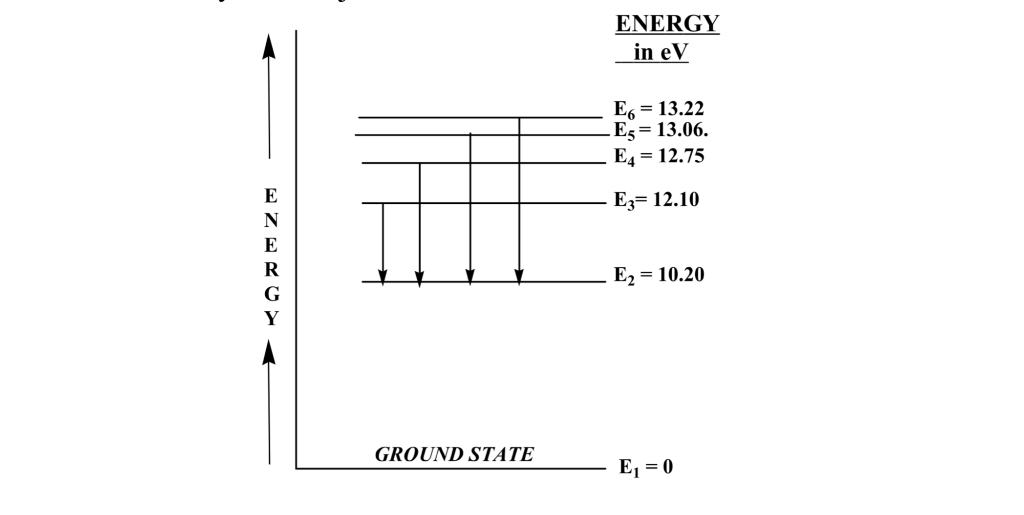
5 10 Draw Energy Level Diagram For Hydrogen Atom Showing At Least Four Lowest Energy Levels Show The Transitions Responsible For Emission Of Balmer Series 0 28 0 38 0 54 086 4 Energy Ev 1 5 1 3 W W Visible Visible 3 5 2 Balmer Series

5 10 Draw Energy Level Diagram For Hydrogen Atom Showing At Least Four Lowest Energy Levels Show The Transitions Responsible For Emission Of Balmer Series 0 28 0 38 0 54 086 4 Energy Ev 1 5 1 3 W W Visible Visible 3 5 2 Balmer Series
Draw Energy Level Diagram For Hydrogen Atom Showing First Four Energy Levels Corresponding Sarthaks Econnect Largest Online Education Community

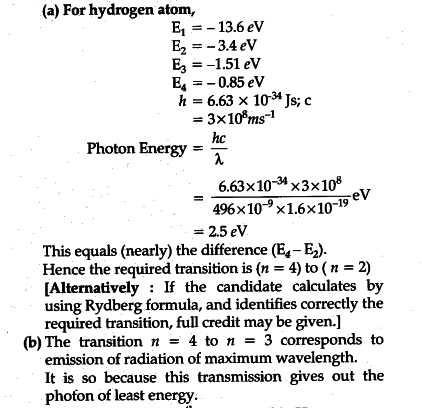
2 The Figure Shows Energy Level Diagram Of Hydrogen Atom Cbse Aic 2015 I Find Out The Transition Which Results In The Emission Of A Photon Of Wavelength 496 Nm Ii Which
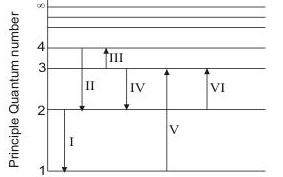
The Figure Shows An Energy Level Diagram For The Hydrogen Atom Several Transition Are Marked As I Ii Iii The Diagram Is Only Indicative And Not To Scale Q The Wavelength Of


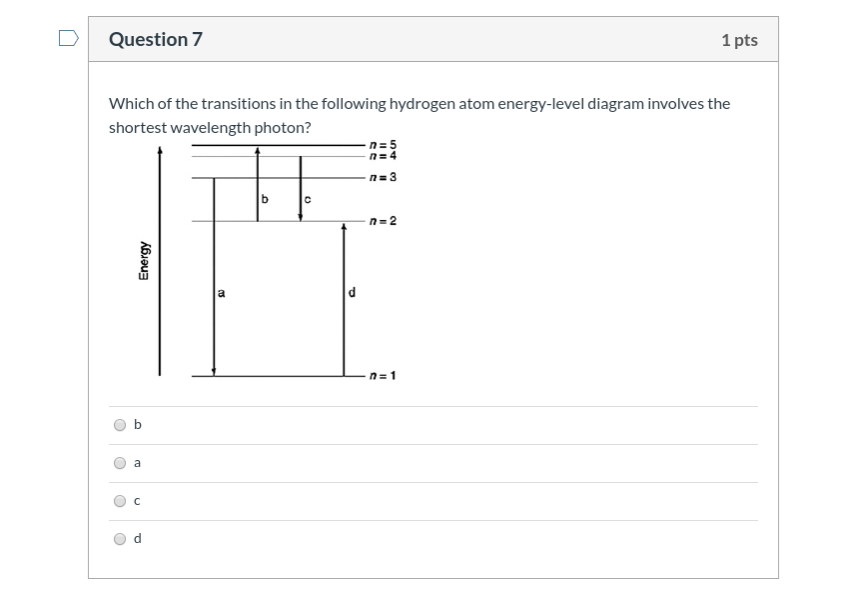

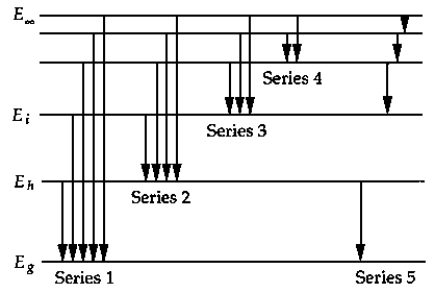
















Comments
Post a Comment