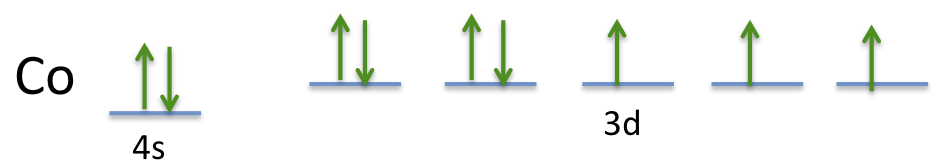
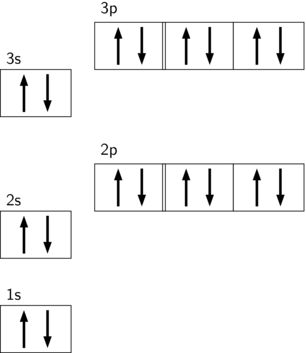
38 orbital diagram for cobalt
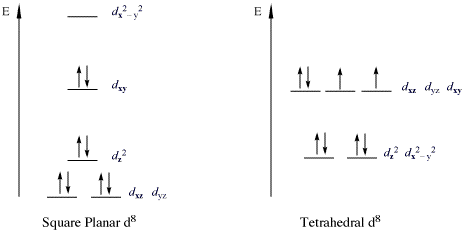
The O 2 unit bridges the two cobalt centers in an end-on bridging fashion (Figure 3) and coordinated DMSO molecules complete the octahedral coordination sphere of each of the Co centers. If we consider the MO diagram of O 2 and d-orbital splitting diagram for [Co(salen)] 2, we can understand why the 2:1 O 2 adduct is favored (Figure 4). Cobalt atomic orbital and chemical bonding information. There are also tutorials on the first thirty-six elements of the periodic table. Check out the blackboard. That box on the left has all of the information you need to know about one element. It tells you the mass of one atom, how many ...
The element is Cobalt (Co)Cobalt, which is classified as a metal, has 27 electrons in its orbital diagram. Its chemical symbol is Co and its atomic number is 27.Cobalt. What is an element that has ...
Orbital diagram for cobalt
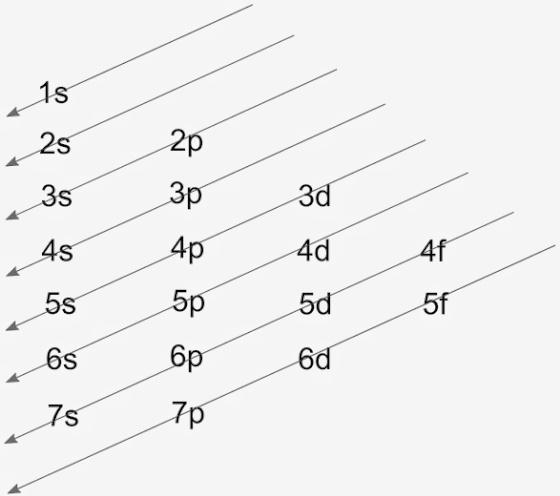
Orbital Diagrams. Another way to represent the order of fill for an atom is by using an orbital diagram often referred to as "the little boxes": The boxes are used to represent the orbitals and to show the electrons placed in them. The order of fill is the same but as you can see from above the electrons are placed singly into the boxes before ... Answer (1 of 9): Transition metals, when losing electrons, first lose s electrons and then d electrons. Here are electron configurations of Co, Co+, Co2+ and Co3+: Co [Ar] 3d7 4s2 Co+ [Ar] 3d7 4s1 Co2+ [Ar] 3d7 Co3+ [Ar] 3d6 <--- answer to your question Example showing how to draw orbital diagrams. Also Cobalt orbital diagram example problem.For more chemistry help videos and practice worksheets go to:https...
Orbital diagram for cobalt. Get the detailed answer: Write the orbital diagram for the ground state of cobalt. The electron configuration is [Ar]3d74s2. What is the electron configuration for cobalt z=27? Why? Chemistry Electron Configuration Electron Configuration. 1 Answer anor277 Apr 8, 2018 Well, we got 27 electrons to distribute according to the aufbau scheme... Explanation: And so... #underbrace(1s^(2 ... Answer (1 of 3): Orbital Diagrams Many times it is necessary to see all the quantum numbers in an electron configuration, this the purpose of the orbital diagram. In addition to listing the principle quantum number, n, and the subshell, ℓℓ, the orbital diagram shows all the different orientation... May 10, 2021 · In this case of Cobalt there are 27 electrons which are present in 4 orbits and their distribution on the orbit that is electronic configuration can be written as: 1s22s22p63s23p63d74s2. What is the electronic configuration of 27? Electronic Configuration of First 30 Elements with Atomic Numbers
cobalt 3+? 3. 3+Using your answer from Question 2, draw a d-orbital splitting diagram for low-spin Co similar to the one given for Cr3+ 3+in the introduction to this experiment. Almost all Co complexes are low-spin, with a minimum number of unpaired electrons. (Refer to your text for further explanation.) 4. Orbital Filling Diagrams •Each box represents an orbital which can hold a max of 2 e- •Aufbau principal –each electron occupies the lowest energy orbital available; German for “build up” •Electrons are notated with an arrow –Up arrow goes first then, down arrow –Arrows represent the opposing spin of electrons 5.2 Quantum Theory & The Atom This chemistry video tutorial provides a basic introduction into orbital diagrams and electron configuration. It explains how to write the orbital diagram n... The Lewis dot diagram for Platinum is a diagram showing bonds & electrons of the Platinum atom within a molecule. Nobody will be able to draw you a diagram here, as this is a text-only answer board. Use Google Image Search if you want to actually see the Lewis diagram for Pt.Cobalt Bohr ModelLewis dot diagram for cobalt.
When Cobalt loses 2 electrons to become $\ce{Co^{2+}}$ it loses the electrons which are in $4s^2$, not the ones in $3d^7$ because the electrons in $4s^2$ have a high reactivity. So $\ce{Co^{2+}}$ still contains 7 electrons in it's valence shell. The new configuration of $\ce{Co^{2+}}$ is $1s^2\ 2s^2\ 2p^6\ 3s^2\ 3p^6\ 3d^7$. Draw and explain the orbital diagram for cobalt (Z = 27). Cobalt: Cobalt is a transition metal element found in period 4 and group 9 of the periodic table. It tends to form colored compounds and ... Orbital Diagram. 1s ... Remains hard up to 982°C. Radioactive cobalt-60 is used in cancer therapy. Sources Occurs in compounds with arsenic, oxygen and sulfur as in cobaltine (CoAsS) and linneite (Co3S4). Pure cobalt is obtained as a byproduct of refining nickel, copper and iron. Solved Fill in the orbital energy diagram for the | Chegg.com. Science. Chemistry. Chemistry questions and answers. Fill in the orbital energy diagram for the cobalt (III) ion. 3d 4s 3p E 3s 2p 1s The lowest E levels are already filled in for you.
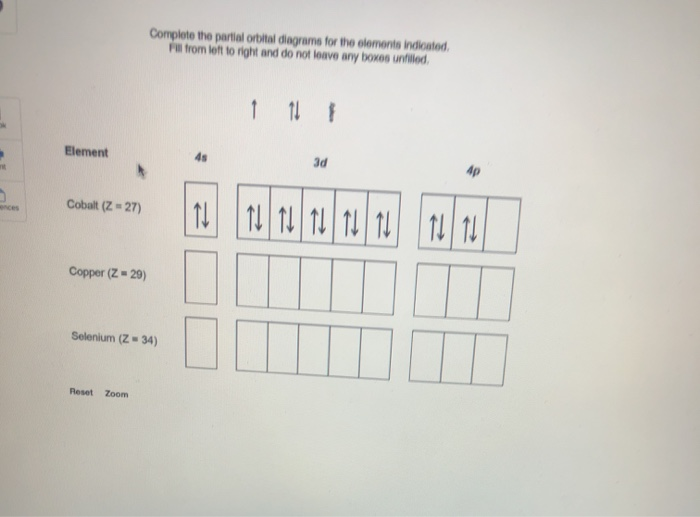
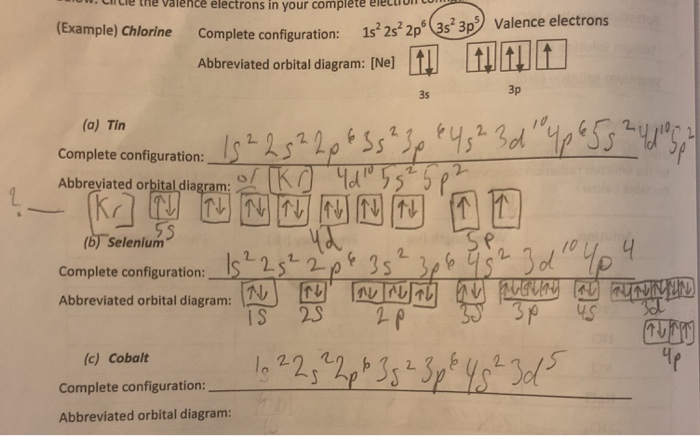
Complete the partial orbital diagrams for the elements Indicated. Fill from left to right and do not leave any boxes unfilled. Element Cobalt (Z = 27) Copper (Z = 29) Selenium (Z = 34) Question: Complete the partial orbital diagrams for the elements Indicated. Fill from left to right and do not leave any boxes unfilled.
Cobalt has been known and used by people for its beautiful colouring and pigment properties as far back as 2500BC. Egyptian cobalt blue paints and Prussian cobalt oxide necklaces have been dated back to this time while cobalt glass has been found in a Greek vase dated at 100 BC.
How Many Unpaired Electrons Are In A Gaseous Co 3 Ion In Its Ground State The Answer Is 4 But I Don T Understand How Could Someone Please Explain Thank You Socratic
The 1s orbital at the bottom of the diagram is the orbital with electrons of lowest energy. The energy increases as we move up to the 2 s and then 2 p , 3 s , and 3 p orbitals, showing that the increasing n value has more influence on energy than the increasing l value for small atoms.
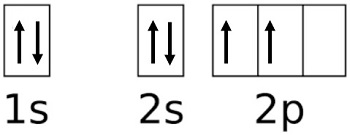
Terms in this set (20) How many boxes are required to depict the 4f orbitals? 7. The orbital diagram represents what element? (1s2 2s2 2p6 3s2 3p6 4s) Potassium. What atom will have unpaired 2p electrons? O (oxygen) Orbitals that have the same energy are called?
Orbital notation is similar to electron configuration. Cobalt's electron configuration is: 1s2 2s2 2p6 3s2 3p6 4s2 3d7 The simplified configuration is: [Ar] 4s2 3d7 A link to the Wikipedia article ...
The complete electron configuration of cobalt is 1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^7. This configuration includes the argon core ( [Ar]:1s^2 2s^2 2p^6 3s^2 3p^6). These electrons are core electrons because they are stable and do not take part in bonding. The remaining electrons are called valence because they do take part in bonding.
Potassium's atomic number is 19. This means that every atom of potassium has 19 protons in its nucleus. In a neutral atom, the number of protons is equal to the number of electrons. So the electron configuration of potassium will involve 19 electrons. The full electron configuration of potassium is "1s"^2"2s"^2"2p"^6"3s"^2"3p"^6"4s"^1". The noble gas notation is "[Ar]4s"^1".
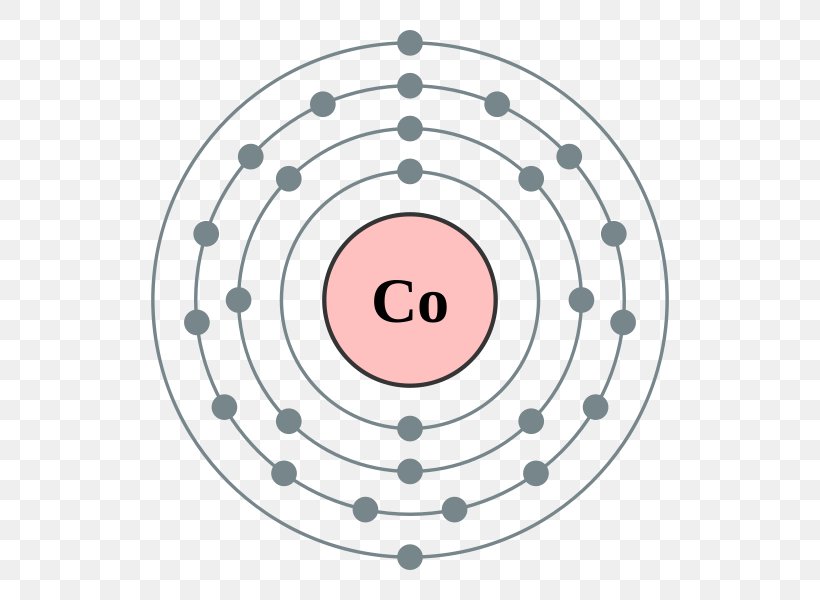
Answer and Explanation: The electron configuration of cobalt is 1s2 2s2 2p6 3s23p6 3d7 4s2. Cobalt is in the first row of the d-block of elements. The 4s orbital is filled. Similarly, it is asked, how many orbitals are in cobalt? Cobalt is a transition metal in the fourth period that is slowly filling up its third shell with electrons. Cobalt ...
Orbital Diagram for Cobalt. orbital diagram example and practice cobalt problem example showing how to draw orbital diagrams also cobalt orbital diagram example problem for more chemistry help videos and practice how to draw the orbital diagram of cobalt i think this question violates the munity guidelines chat or rant adult content spam insulting other members show more i think this
The orbital diagram for the atom of Cobalt is shown below. Cobalt has a total of 27 electrons which are contained in 1s, 2s, 2p, 3s, 3p, 4s and 3d sub...
Electron Configurations How To Write Out The S P D F Electronic Arrangements Of Atoms Ions Periodic Table Oxidation States Using Orbital Notation Gce A Level Revision Notes
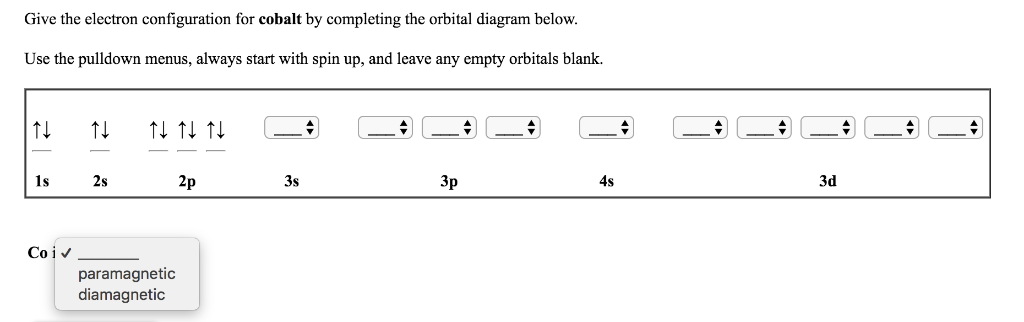
Which shows the correct orbital diagram for Cobalt? answer choices . alternatives . answer explanation . Tags: Topics: Question 10 . SURVEY . Ungraded . 30 seconds . Report an issue . Q. What element's orbital notation is pictured here? answer choices . Ar. Br. Cu. Cl <p>Ar</p> ...
Nov 01, 2021 · Orbital diagram of Cobalt (Co) 28: Orbital diagram of Nickel (Ni) 29: Orbital diagram of Copper (Cu) 30: Orbital diagram of Zinc (Zn) 31: Orbital diagram of Gallium (Ga) 32: Orbital diagram of Germanium (Ge) 33: Orbital diagram of Arsenic (As) 34: Orbital diagram of Selenium (Se) 35: Orbital diagram of Bromine (Br) 36: Orbital diagram of ...

Or Exam 2 Material Saved Omplete The Partial Orbital Diagrams For The Elements Indica Fill From Left To Right And Homeworklib
Example showing how to draw orbital diagrams. Also Cobalt orbital diagram example problem.For more chemistry help videos and practice worksheets go to:https...
Answer (1 of 9): Transition metals, when losing electrons, first lose s electrons and then d electrons. Here are electron configurations of Co, Co+, Co2+ and Co3+: Co [Ar] 3d7 4s2 Co+ [Ar] 3d7 4s1 Co2+ [Ar] 3d7 Co3+ [Ar] 3d6 <--- answer to your question
Orbital Diagrams. Another way to represent the order of fill for an atom is by using an orbital diagram often referred to as "the little boxes": The boxes are used to represent the orbitals and to show the electrons placed in them. The order of fill is the same but as you can see from above the electrons are placed singly into the boxes before ...
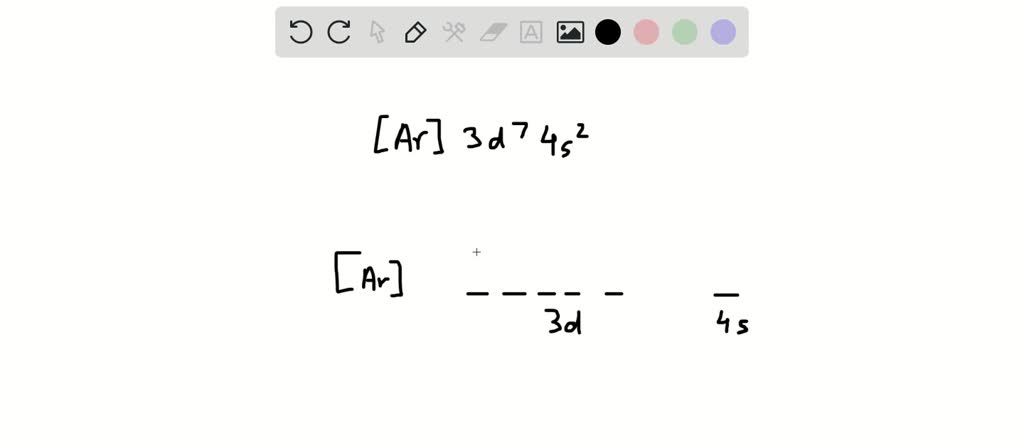
Solved Write The Orbital Diagram For The Ground State Of Cobalt The Electron Configuration Is Mathrm Ar 3 D 7 4 S 2

Solved In The Bohr Model Of The Atom Electrons Occupy Fixed Orbits Around The Nucleus Called Energy Levels However In The Quantum Mechanical Modcl Of The Atom Clectrons Occupy Obitals Orbitals Are Grouped












/800px-Orbital_representation_diagram.svg-589bd6285f9b58819cfd8460.png)




Comments
Post a Comment